Facts from ECB CH3, Energy, Catalysis, and Biosynthesis
| 876 wordsHere are some facts I found interesting from the third chapter of Essential Cell Biology.
The Use of Energy by Cells
- Metabolism might be defined as the sum total of all the chemical reactions an organism needs to carry out to survive and reproduce. Catabolic pathway breaks down food into smaller molecules, generating energy. Anabolic pathways use energy to drive the synthesis of the molecules that form the cell.
- Cells must pump against entropy in order to stay alive, thus they must release waste heat.
- Photosynthetic organisms use sunlight to synthesize organic molecules.
- Oxidation can be though thought of as controlled burning.
- Acronym: LEO says GER. Losing electron (is) oxidation, gaining electron (is) reduction.
- An increase in C-H bonds is a reduction, a decrease is oxidation.
Free Energy and Catalysis
- Enzymes can make reactions cost less energy, but not zero.
-
Chemical reactions proceed in the direction that causes a loss of free energy. Free energy can be thought of as an accounting trick that takes into account the entropy of everything involved when you want to divide the world into “inside the system of interest” and “outside the system of interest”.
- Activation energy is needed to start spontaneous reactions. Observe how flammable materials don’t spontaneously burst into flames.
-
Enzymes bind to molecules and hold them in a way that reduces the activation energy needed. This can make reactions much faster than they would happen natrually, up to 10^14 times.
-
You can get energetically unfavorable reactions (positive ΔG) to happen by coupling them to reactions with negative ΔG, thus making the net reaction energetically favorable.
- Cells at equilibrium are dead.
- The equilibrium constant K is the ratio of the concentrations at equilibrium (products divided by reactants). It is directly proportional to ΔG°.
- For sequential reactions, the changes in free energy are additive. If X->Y is unfavorable and Y->Z is more favorable than the previous reaction was unfavorable, coupling the reactions X->Y->Z can produce a favorable reactions; Y->Z acts as a siphon to pull the entire reaction along.
- Small things are fast. A typical enzyme can capture and process a thousand substrate molecules every second. A small organic molecule takes only one-fifth of a second to diffuse a distance of 10 µm. The most abundant substrates are present in cells at concentrations of about 0.5 mM. This translates to 500k collisions with the enzyme every second.
- Enzymes can also couple unfavorable and favorable reactions together to form favorable reactions.
- The association of enzyme and substrate is stabilized by the formation of multiple weak bonds. If the two colliding molecules don’t mesh together, very few bonds are formed and they are rapidly torn apart by thermal noise.
- enzymes must symmetrically lower the activation energy for reactions, because the same set of noncovalent bonds are formed in either case.
Activated Carriers and Biosynthesis
-
Food energy must be used to drive the reactions in a cell. In most cases, the energy is first stored as chemical bond energy in activated carriers. The formation of an activated carrier is coupled to an energetically favorable reaction.
- ATP (adenosine 5’-triphosphate) is the most widely used activated carrier. It gets synthesized from adenosine 5’diphosphate (ADP) in a reaction that adds a phosphate group.
- The large favorability of removal of a phosphate group from ATP is because it removes an unfavorable repulsion between adjacent negative charges and the inorganic phosphate ion forms favorable hydrogen bonds with water.
- Energy stored in atp is often harnessed to join two molecules together. A common type of reaction that is needed is joining A-OH and B-H in a unfavorable condensation reaction. ATP hydrolysis can make this work by having A-OH first converted to a higher energy intermediate compound, which then reacts with B-H to form A-H. One way this happens is ATP gives it’s phosphate to A-OH to form A-O-PO_3.
- NADH and NADPH are both activated carriers of electrons. NADH = nicotinamide adenine dinucleotide. NADPH = nicotinamide adenine dinucleotide phosphate. Both carry energy in form of two high-energy electrons and a proton, which form a hydride ion (H-), which can be passed onto donor molecules.
- Chemically, NADPH and NADH only differ in a single phosphate group, which doesn’t affect their abilities to store energy. However, this difference means they have a different shape, so they can be controlled separately. NADPH operates with enzymes that catalyze anabolic reactions, which use energy to drive the synthesis of the molecules that form the cell. NADH has a special role as an intermediate in the catabolic system that generates ATP. Inside cells, the ratio NAD+/NADH is high, so it can accept electrons easily in catabolic pathways; in contrast, the ratio NADPH/NADP+ is high, so NADPH can easily donate electrons for anabolic pathways.
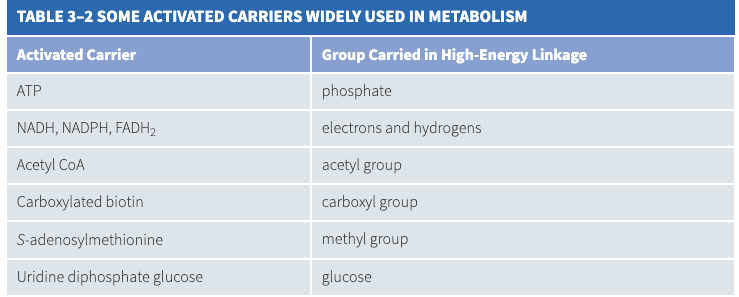
- Here is a table listing various activated carriers.
- In activated carriers, the transferable group only makes up a small portion of the molecule. The rest consists of a large organic portion that serves as a handle, allowing different activated carriers to be controlled separately, creating a wire-network of sorts.
- The ΔG of ATP -> ADP is generally between -46 and -54 kJ/mole in typical conditions inside a cell.